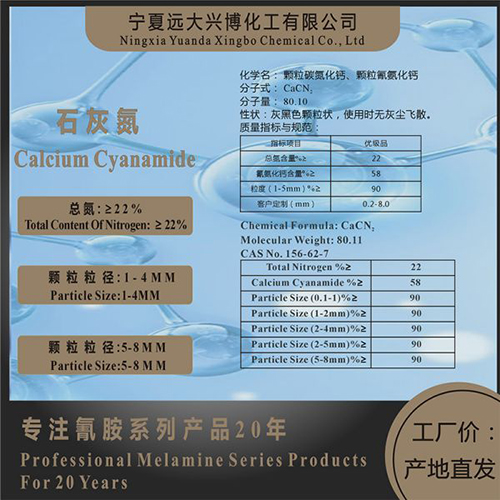
Chemical Formula: CaCN2
Molecular Weight: 80.11
CAS No. 156-62-7
Total Nitrogen %≥ | 22 |
Calcium Cyanamide %≥ | 58 |
Calcium Carbide %≤ | 0.30 |
Particle Size (0.1-1)%≥ | 90 |
Particle Size (1-2mm)%≥ | 90 |
Particle Size (2-4mm)%≥ | 90 |
Particle Size (2-5mm)%≥ | 90 |
Particle Size (5-8mm)%≥ | 90 |
Specificity: | Grey and black granular. Easy to turn into powder in wet environment. Its water solution is alkaline. |
Application: | A kind of pesticide with low toxicity and no residuals and also a kind of slow-release green pesticides and fertilizers with the efficiency of pesticide, germicide and weedicide. It can raise earth temperature; adjust alkalinity and acidity of the soil. Applying it can speed up decomposing the roots, stems and straws of the plants left in the soil and then they can be changed into organic matter. Meanwhile, it can inhibit nitrification. The most outstanding advantages of the product are its dual efficiency of pesticide and fertilizer, no flying when applying and no environmental pollution.It can also be made into cored wire for steel production |
Lime nitrogen is a mixture of calcium cyanamide, calcium oxide and other insoluble impurities. It is grayish black and has a special odor. It is an alkaline fertilizer and one of the main raw materials of carbendazim pesticide with high efficiency and low toxicity. It can be used as herbicide, bactericide and insecticide, and can be used to produce dicyandiamide, melamine and cyanide melt.
Application: it has the dual effects of pesticide and fertilizer. It has the effect of general nitrogen fertilizer and has the effects of killing insects, sterilizing, weeding and improving ground temperature. It can promote the roots and stems of dead plants to mature in advance and convert them into organic fertilizer. It has the effect of sterilization for greenhouses. It is a good acidic soil conditioner with slow failure. It will not cause environmental pollution and harm to human body during sowing, Its most prominent feature is its comprehensive effect.
Packaging, storage and transportation: PE bags, with a net weight of 20kg / 25kg. Handle with care during shipment. Store in a cool, well ventilated and dry warehouse, pay attention to moisture and do not stack against the wall.
Please note when using: when applying lime nitrogen, wear masks and work clothes, stay away from livestock living areas, and do not drink alcoholic drinks within 24 hours to avoid adverse chemical reactions such as chest tightness and suffocation. In case of poisoning or accidental ingestion, do not induce vomiting. Drink more water. In case of contact with skin, take off contaminated clothes, wash skin, accidentally enter eyes, open eyes and rinse with plenty of water, please see a doctor as soon as possible.
Chemical Properties
Calcium cyanamide is a blackish-gray, shiny crystalline material or powder.
Physical properties
Pure product is a colorless, hexagonal crystal or white powder. Commercial grade material may be grayish-black powder or lump (the color is due to presence of calcium carbide and other impurities); density 2.29 g/cm3; melts around 1,340°C; sublimes around 1,150 to 1,200°C on rapid heating; reacts with water.
Definition
ChEBI: The calcium salt of cyanamide, formed when calcium carbide reacts with nitrogen
Definition
calcium cyanamide: A colourlesssolid, CaCN2, which sublimes at1300°C. It is prepared by heating calciumdicarbide at 800°C in a streamof nitrogen:
CaC2(s) + N2(g) → CaCN2(s) + C(s)
The reaction has been used as amethod of fixing nitrogen in countriesin which cheap electricity isavailable to make the calcium dicarbide(the cyanamide process). Calciumcyanamide can be used as afertilizer because it reacts with waterto give ammonia and calcium carbonate:
CaCN2(s) + 3H2O(l) → CaCO3(s) +2NH3(g)
It is also used in the production ofmelamine, urea, and certain cyanidesalts.
Production Methods
Calcium cyanamide was first produced commercially around 1900 as a fertilizer. The process of making calcium cyanamide involves three raw materials—coke, coal, and limestone— plus nitrogen. The limestone (calcium carbonate) is burned with coal to produce calcium oxide. The calcium oxide is then allowed to react with amorphous carbon in the furnace at 2000°C with the formation of calcium carbide (CaC2). Finely powdered calcium carbide is heated to 1000°C in an electric furnace into which pure nitrogen is passed. It is then removed and uncombined calcium carbide removed by leaching.
Preparation
Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1,000°C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.
CaC2 + N2 → CaCN2 + C (ΔHƒ°= –69.0 kcal/mol at 25°C)
General Description
A colorless to gray, odorless solid. May cause illness from ingestion. May irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers.
Air & Water Reactions
Depending on the calcium carbide content, the cyanamide reacts with water (moisture from air or soil) to produce acetylene and hydrated calcium oxide or calcium hydroxide. Absorption of water during handling or storage of technical calcium cyanamide may cause explosion [Pieri, M. Chem. Abs. 46, 8335 1952].
Reactivity Profile
When hydrated CALCIUM CARBIDE generates salts of calcium that are basic and are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Fire risk with moisture or combined with calcium carbide. Skin, eye, and upper respiratory tract irritant. Questionable carcinogen.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Agricultural Uses
Calcium cyanamide (CaCN2) is a dark colored, granulated material containing around 21 % nitrogen. Its dark color is due to the presence of calcium carbide. Calcium cyanamide is produced by heating a mixture of limestone with coal in a nitrogen atmosphere.
Generally, the process is carried out in three steps. In the first step, calcium carbonate (limestone) is decomposed at about 1100°C.
In the second step, calcium oxide (CaO) and coke (or coal) are heated in an electric furnace to produce calcium carbide.
The final step involves heating the powdered calcium carbide at about 1100°C with pure nitrogen (produced by liquefaction of air and fractional distillation) to produce calcium cyanamide.
The fertilizer-grade calcium cyanamide contains 21 % nitrogen, 11 % calcium, 11 % free carbon, 5% oil, 2 to 4% water and oxides of aluminum, iron and silicon. In the presence of moisture and air, calcium dicyandiamide (a poisonous compound) is formed. It distinctly leaves alkalinity in the soil equivalent to 1.3 kg calcium carbonate (CaCO3) per 0.45 kg of nitrogen applied. At pH 7 or below, calcium dicyandiamide is converted into urea and lime within one week of its being in the soil.
When dry, calcium cyanamide is dusty but it is generally used as granules. It is poisonous, irritating to the skin and used as a pesticide, fertilizer and defoliant in cotton. It is as good a fertilizer as sodium nitrate or ammonium sulphate, but not as fast acting.
Calcium cyanamide is an excellent weed killer, especially for tobacco plants, when applied 2 to 3 weeks before sowing. It is also used for producing melamine, urea and certain cyanide salts.
Safety Profile
Poison by ingestion, inhalation, sh contact, intravenous, and intraperitoneal routes. Moderately toxic to humans by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. The fatal dose, by ingestion, is probably around 20 to 30 g for an adult. It does not have a cyanide effect. Calcium cyanamide is not believed to have a cumulative action. Flammable. Reaction with water forms the explosive acetylene gas. When heated to decomposition it emits toxic fumes of NOx and CN-. See also CALCIUM COMPOUNDS, AMIDES, and CYANIDE
Potential Exposure
Calcium cyanamide is used in agriculture as a fertilizer, herbicide; defoliant for cotton plants; and pesticide. It is also used in the manufacture of dicyandiamide and calcium cyanide as a desulfurizer in the iron and steel industry; and in steel hardening.
Carcinogenicity
Calcium cyanamide was weakly mutagenic in Salmonella typhimurium strain TA1535 and nonmutagenic in strain TA100.
Shipping
UN1403 Calcium cyanamide with .1% calcium carbide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material
Incompatibilities
Commercial grades of calcium cyanamide may contain calcium carbide; contact with any form of moisture solutions may cause decomposition, liberating explosive acetylene gas and ammonia. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May polymerize in water or alkaline solutions to dicyanamide. Contact with all solvents tested also causes decomposition